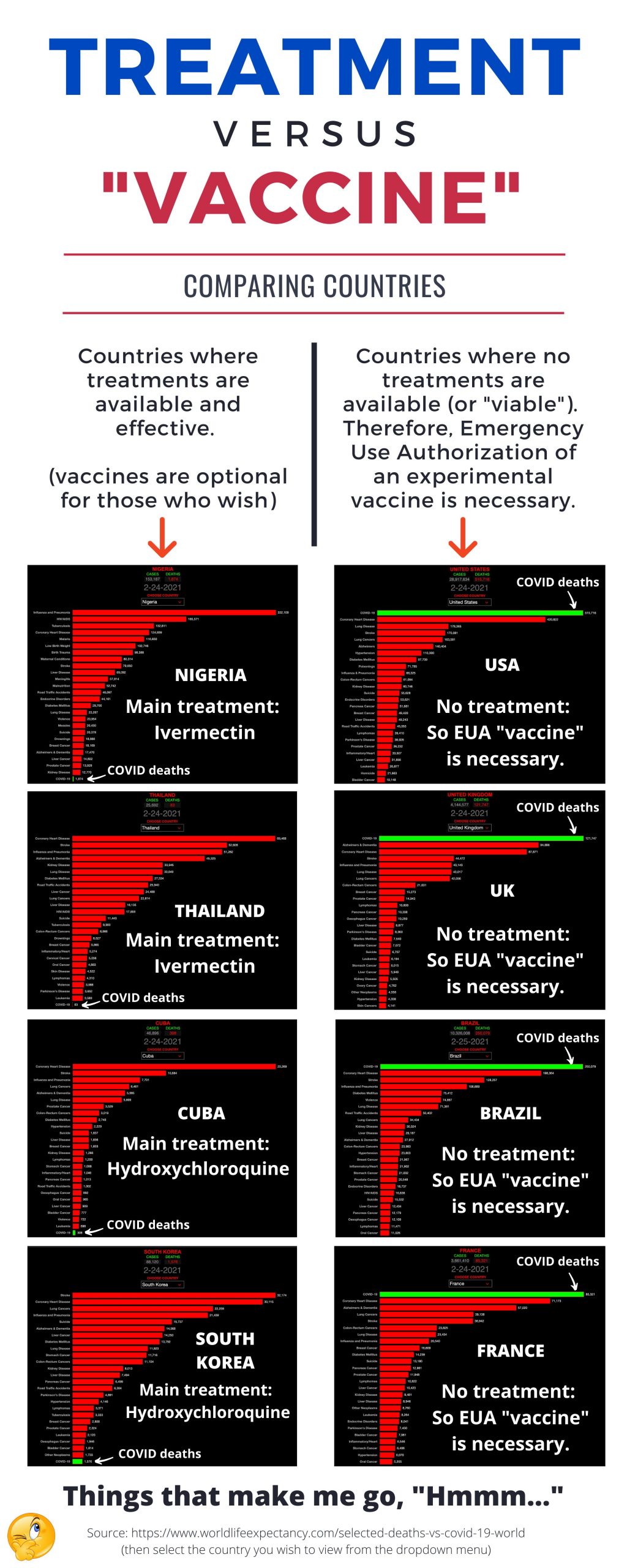
Here are some facts to understand:
- Emergency Use Authorization (EUA) is issued only when there is no treatment for a disease.
- If there is a “viable” treatment, EUA cannot be issued.
- Once EUA is issued, experimental products can be “authorized” by the FDA (but this is very different from being “approved”).
- Products authorized under EUA are immune from lawsuits and liability for injuries, any and all harm, and death. The vaccine companies are already immune from lawsuits and liability, thanks to the Vaccine Injury Act of 1986, so with the EUA, both the vaccine manufacturers and the government are 0% liable if it all goes horribly wrong.
There are, however, several viable and extremely effective treatments for preventing the infection of the Novel Coronavirus and resulting COVID symptoms.
This post will discuss the top two treatments used in other countries, and the success rates displayed in the graphic below, compared to the countries who claim those treatments are not viable, so the experimental “vaccines” must be used under EUA.
Not to mention, the definition of vaccine has been changed because the current products (as of this post on 2/25/21) for COVID-19 operate in a completely different way than all the vaccines of the past.
Things that make me go, “Hmmm…”